9 Jan 2017
CBSE Class 11 Chemistry Notes : Ionic Equilibrium
The equilibrium established between the unionised molecules and the ions in the solution of weak electrolytes is called ionic equilibrium.
e.g.,
CH3 COOH ⇔ CH3COO– + H–
Electrolytes
Chemrcal substances which can ccnduct electricity in their aqueous stare or tn molten state are called electrolytes. The conduction of current through electrolyte is due to the movement of ions.
1. Strong Electrolytes
Electrolytes which dissociate almost completely into constituent ions in aqueous solution are known as strong electrolytes. e.g.. all salts (except HgCl2, CdBr2)’ mineral acids like HCl, H2)SO4, HNO3 etc.. and baseslike NaOH. KOH. etc.
2. Weak Electrolytes
Electrolytes which dissociate to a lesser extent in aqueous solution are called weak electrolyte. All organic acids (except sulphonic acids), and bases like NH3. NH4OH, amines etc.
Degree of Ionisation or Degree of Dissociation (α)
It is the fraction of the total number of molecules which ionise (dissociate)into constituent ions.
α = (number of molecules ionised or dissociated/total number of molecules taken)
For strong electrolytes,
α = 1
For weak electrolytes
α < 1
Values of the degree of dissociation (ex)depends upon the following factors
- nature of solute
- nature of solvent
- concentration
- temperature
Ostwald’s Dilution Law
According to Ostwald. the degree of dissociation (ex)of weak electrolyte is inversely proportional to the square root of the molar concentration of the solution.
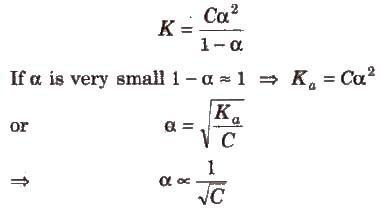
Hore K is dissociation constant and C is molar concentration of the solution.
Acids and Bases
Earlier Definitions of Acids and Bases
Earlier definitions of acids and bases was given by Robert Boyle, who classified them on the basis of their properties. According to him, acids are the substance which have sour taste. turns blue litmus red. liberate hydrogen with metals, conduct electricity in aqueous solution and neutralise bases.
Bases are the substance which have bitter taste, turns red litmus blue, soapy to touch, conduct electricity in aqueous solution and neutralise acids.
Arrhenius Concept of Acids and Bases
- Acid is a chemical substance which dissociates in aqueous solution to give hydrogen ions (H+) or hydronium ions (H3O+).
- Base is a chemical substance which dissociates in aqueous solution to give hydroxyl ions (OH–).
- Arrhenius theory fails to explain the acidic and basic behaviour in non-aqueous solutions. It cannot explain the acidic character of A1Cla. BFa and basic character of NH3, PH3, etc.
Bronsted Concept of Acids and Bases
Acid is a chemical substance that can donate a proton (H+) to some other substance and a base is a chemical substance that can accept a proton from other substance. Thus, an acid is a proton donor (protongenic) and a base is proton acceptor (protopbilic).
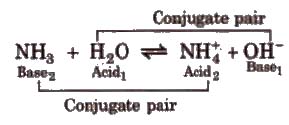
Strong acid has weak conjugate base and weak acid has strong conjugate basco Strong base has weak conjugate acid and weak base has strong conjugate acid.
HClO4 is the strongest while HCN is the weakest hydracid known.
CsOH is the strongest base known.
Amphoteric or arnphiprotic substance or ampholytes are the substance which act as an acid as well as a base, e.g.• water acts as an acid with NH3 and a base with acetic acid.
The order of acidic strength of some acids is
HClO4 > HBr> H2SO4 > HC1> HNO3
Greater the Ka value of an acid (or lesser the pKa), stronger is the acid. Similarly. greater the Kb (or lesser the pKb) of a base. stronger is the base.
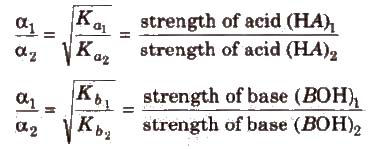
Levling Effect
The adds like HClO4 H2SO4, HNO3 etc. react with water almost completely to form H3O+ ions.Therefore,all the strong acids in aqueous solutionsappear equally strong and their relative strengths in aqueous solution cannot be compared. Since H3O+ is the strongest acid in water. the strength of above acids come down to the level of H3O+ strength in water. Similarly.strong bases like NaOH. KOH. Ba(OH)2 come down to the strength of OH– ion in water.
This is called levling effect.
Lewis Concept of Acids and Bases
Lewis acid is a chemical substance which can accept a pair of electrons,
e.g.,
- Molecules with incomplete octet of central atom like AlCl3 ,BeCl2, MgCl2, etc.
- Simple cations like Ag+, Na+, etc.
- Molecules in which the central atom has vacant d-orbital, e.g.,SF4, SnC14 PF3 etc.
Lewis base is a chemical substance which can donate a pair of electrons. e.g.,
- Neutral molecules containing lone pairs like NH3, RNH2, ROH etc.
- Negatively charged species like CN, Cl. OH, etc.
- In coordination complexes, the ligands act as Lewis base.
Limitations of Lewis Concept
- It does not explain the behaviour of protonic acids such as HCl, H4SO4, HNO3 etc.
- It does not predict the magnitude of relative strength of acids and bases.
All Bronsted-Lowry’s acids are Lewis acids while acids need not be Bronsted-Lowry’s acids.
The Ionization Constant of Water Ionic product is the product of the concentration of hydronium ions and hydroxyl ion in pure water, which remains constant at a particular temperature. It is symbolized by Kw. At 298 K, ionic product of water (KW) is given as KW: = [H3O+] [OH–] = 1 x 10-14 mol2L–.
The value of Kw increases with increase in temperature.
The pH Scale
pH is defined as the negative logarithm of hydrogen ion concentration.
pH = – log [H+] and [H+J = lO-pH
Total [H+] or [OH–] in a mixture of two strong acids or bases = (ΣNV/ΣV)
Similarly, negative logarithm of hydroxyl ion concentration is pOH.
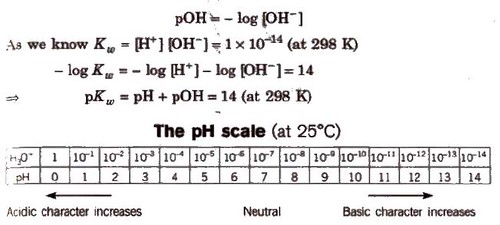
pH value of an acid having H+ concentration less than 10-7, is always in between 6 and 7. For 10-8 N HCl solution. it is 6.958.Similarly for 10-8 NaOH solution, the pH is 7.04 (because basic solutions always have pH 77.)
pH of solution is accurately measured by pH meter or emf method or roughly by pH paper or indicator paper.
(PH can be zero in 1 N Hel solution or it can be negative for more. concentrated solution like 2N, 3N, lON, etc,
pH range for some important substances are :
Gastric juice = 1 – 3
Vinegar = 2.4 – 3.4
Tears – 7.4
Human urine – 4.8 – 8.4
Blood plasma – 7.3 – 7.4
Boil water – 6.5625
Vinegar = 2.4 – 3.4
Tears – 7.4
Human urine – 4.8 – 8.4
Blood plasma – 7.3 – 7.4
Boil water – 6.5625
Dissociation Constant of Weak
Acid and Weak Base
Acid and Weak Base
Let us consider the dissociation of weak acid (HA) as
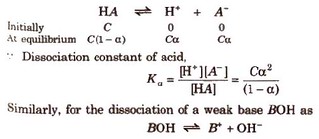
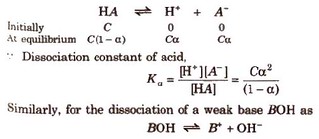
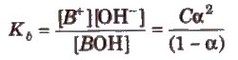
Dissociation constant for polyprotic acids and bases. For a tribasic acid,
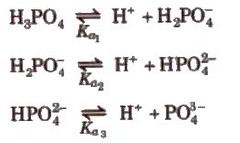
The overall dissociation constant (K) is given as
K = K1 x K2 x K3
where, K1 x K2 x K3

Similarly, for a dibasic acid like H2CO3
pH = pka1 + pka2/2)
Buffer Solution
Solution which resists the change in its pH value by addition of a small amount of acid or a base, is called buffer solution.
- Acidic buffer They have pH value < 7, e.g., CH3COOH/CH3COONa, bone acid/borax.
- Basic buffer They have pH value> 7 e.g., NH4OH/NH34Cl
Buffer system present in blood is H2CO3 + NaHCO3.
Henderson-Hesselbalch Equation
Equation used to calculate the pH of a buffer solution.
(i) For acidic buffer,
pH = pKa + log[salt/acid]
(i) pOH = pKb + log[salt/acid]
and pH = 14 – pOH
Here, pKa = -log Ka, pKb = -log Kb and Ka and Kb are dissociation constants of acid and base.
[salt), [acid] and [base) represent molar concentrations of salt, acid and base respectively.
If addition of a strong acid or base changes the pH of a buffer by/unit, the buffer solution is assumed to destroyed, i.e.,
New pH = pKo ± 1
This means the ratio.
[salt]/[acid) or [salt]/[base] = 10 or (1/10)
Buffer Capacity
It is defined as the number of moles of acid or base added in 1 L of solution Lochange the pH by unity.
Buffer capacity (φ)
= (no. of moles of acids or base added to 1 L of buffer/change in pH)
Salts
These are the product of reaction between an acid and a base.
This reaction is called neutralisation reaction.
This reaction is called neutralisation reaction.
Types of Salts
(a) Normal salts These are obtained by complete neutralisation of an acid with a base, e.g., NaCI, K2SO4, etc.
(b) Acidic salts These are formed by incomplete neutralisation of polybasic acids. e.g., NaHCO3, Na2SO4etc.
(c) Basic salts These are formed by incomplete neutralization of polyacidic base, e.g., Mg(OH)Cl, Bi(OH)2Cl, etc.
(d) Double salts These are formed by the combination of two simple salts and exist only in solid state, e.g., Mohr salt or ferrous ammonium sulphate (FeSO4.(NH4)2SO4.6H2O], alum, etc.
(e) Complex salts These are formed by the combination of simple salts or molecular compounds. These are stable in solid state well as in solutions.
The properties of their solutions are different from the properties of substances from which they have been constituted.
(f) Mixed salts These salts furnish more than one cation or more than one anion when dissolved in water, e.g., Ca(OCl)Cl, NaKSO4, etc.
Salt Hydrolysis
Salts are strong electrolytes and on dissolution in water split up into ions which react with H+ or OH– ions furnished by water yielding acidic or basic solution. The process is known as salt hydrolysis.
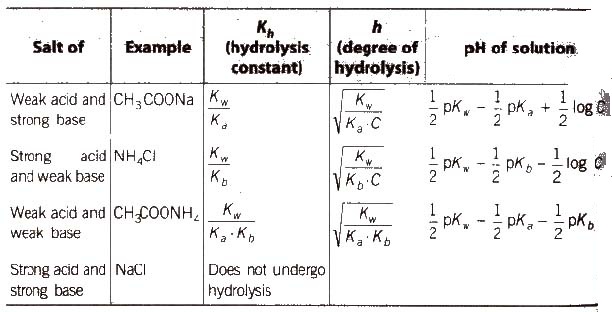
Aqueous solution of salt of strong acid and strong base is neutral Aqueous solution of salt of a weak acid and a strong base is alkaline due to anionic hydrolysis, and aqueous solution of salt of strong acid and a weak base is acidic due to cationic hydrolysis with dilution degree of hydrolysis increases.
Hydrolysis is a reverse process of neutralisation.
Common Ion Effect
It is.defined as the suppression of the dissociation of a weak electrolyte by the addition of a strong electrolyte having some common ion, e.g., degree of dissociation of ammonium hydroxide decreases in the presence of ammonium chloride.
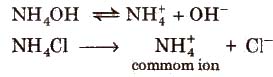
According to Le-Chatelier principle, because of the presence of common ion. degree of dissociation of NH4OH decreases.
Colnmon ion effect is used in
- Purification of common salt
- Salting out of soap
- Qualitative analysis, II group radicals are precipitated out in the presence of HCI which suppress the S2- ion concentration, which is just sufficient to precipitate only II group radicals.
Similarly in group III, NH4OH is added in presence ofNH4Cl to avoid the precipitation of V group radicals.
Isohydric Solutions
If the concentration of the common ions in the solution of two alectrolytes, e.g., OH- ion concentration in Ca(OH)2 and Ba(OH)2 solutions, is same then on mixing them there is no change in degree of dissociation of either of the electrolytes. Such solution are called isohydric solutions.
Solubility Product
It is defined as the product of the concentrations of the ions of the salt in its saturated solution at a given temperature raised to the power of the ions produced by the dissociation of one mole of the salt. It is denoted by Ksp.
Consider the dissociation of an electrolyte AxBy
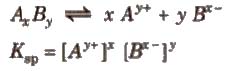
Application of Solubility
1. The concept Product of Ksp helps in predicting the formation of precipitate. In general if
- Ionic product < Ksp,no ppt. is formed.
- Ionic product > Ksp, ppt. is formed.
2. In predicting the solubility of a sparingly soluble salt
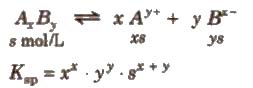
knowing the values of Ksp, x and y, the solubility of the salt can be Computed.
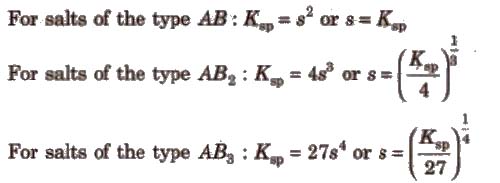
Ksp of AgI is lower than t.hat of Agel. So the former gets precipitated in preference to later.
Distinction Between Solubility Product and Ionic Product

Acid-Base Indicator
An acid-base indicator is a substance which possesses one colour in acid solution and altogether different colour in alkaline medium or the substance which shows colour change with change in pH. The point where the indicator shows a sudden change in colour during the titration is called end point. End point is the point at which the reaction is observed to be complete.
Theory of indicators
(i) Ostwald theory
(ii) Quinonoid theory
(ii) Quinonoid theory
Titration Curves and Indicator Used
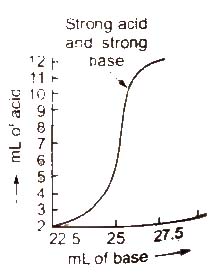
(a) Titration curve for the neurralisation of strong acid vs strong base pH curve of strong acid (say HCl) and strong base
(say NaOH) is vertical over almost the pH range 4-10. So. the indicators phenolphthalein (pH range 8.3 to 10.5). methyl red (pH range 4.4-6.5 and methyl orange (pH range 3.2-4.5) are suitable for such a titration.
b) Titration curve for the neutralisation of strong acid vs weak base pH curve of strong acid (say HCl or H2SO4” or HNO3) with a weak base (say NH4OH) is vertical over the pH range of 4 to 7. So the indicators methyl red and methyl orange are suitable for such a titration.
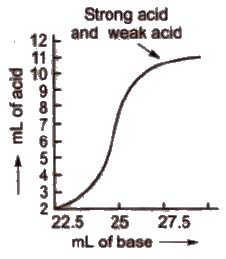
(c) Titration curve for the neutralisation of weak acid vs strong base pH curve of weak acid (say CH3COOH or oxalic acid) and strong base (say NaOH) is vertical over the approximate pH range 7 to 11. So phenolphthalein is the suitable indicator for such a titration.
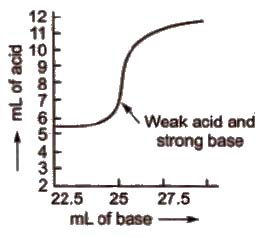
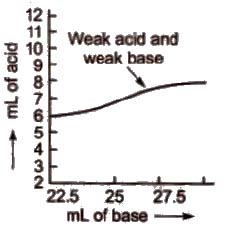
d) Titration curve for the neutralisation of weak acid vs weak base pH curve of weak acid and weak base indicates that there is no vertical part and hence, no suitable indicator can be used for such a titration.